Chiral squaramide-catalysed one-pot enantioselective sulfa-Michael addition/thioesterification of thiols with α,β-unsaturated N-acylated succinimides†
Abstract
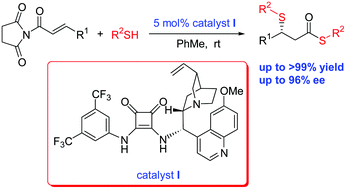
A novel highly enantioselective one-pot dithiolation through sulfa-Michael addition/thioesterification of thiols with α,β-unsaturated N-acylated succinimides catalysed by squaramide has been developed. This organocatalysed reaction proceeded well in high to excellent yields (up to >99%) to afford useful bioactive β-sulfated thioester derivatives with high enantioselectivities (up to 96% ee).

 Please wait while we load your content...
Please wait while we load your content...