Substituent effects on the turn-on kinetics of rhodamine-based fluorescent pH probes†
Abstract
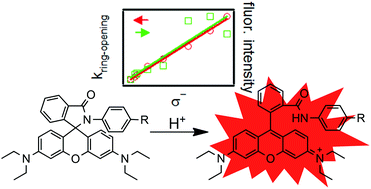
Fluorescent turn-on probes based on a rhodamine spirolactam (RSL) structure have recently become a popular means of detecting pH, metal ions, and other analytes of interest. RSLs are colorless and non-fluorescent until the target analyte induces opening of the spirocyclic ring system, revealing the fully conjugated and highly fluorescent rhodamine dye. Among RSLs opened by acid, we have observed wide variation in the kinetics of the fluorescence turn-on process such that some probes would not be usable in situations where a rapid reading is desired or the pH fluctuates temporally. Herein we present a systematic investigation of the fluorescence turn-on kinetics of RSLs to probe the hypothesis that the reaction rates are influenced by the electronic properties of the spirolactam ring system. A series of 8 aniline-derived RSLs with para substituents ranging from electron-donating to electron-withdrawing was prepared from rhodamine B. The fluorescence turn-on rates are observed to increase by a factor of four as the substituent is tuned from methoxy to nitro. This effect is explained in terms of the destabilization of the reaction intermediate by the substituent. As the reaction rates increase across the series, a concomitant increase in fluorescence intensity is also observed. This result is attributed to an increase in the concentration of the fluorescent form of the dye and is consistent with the expected equilibrium properties of this system. These findings are applied to the design of a faster-reacting and more intensely fluorescent RSL pH probe.

 Please wait while we load your content...
Please wait while we load your content...