Rh-catalyzed allylic C–F bond activation: the stereoselective synthesis of trisubstituted monofluoroalkenes and a mechanism study†
Abstract
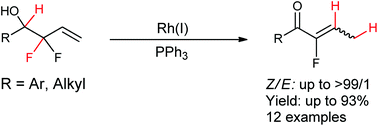
Rhodium-catalyzed allylic C–F bond activation via oxidative addition was found to be a promising approach for the conversion of allylic difluoro-homoallylic alcohols into trisubstituted monofluoroalkenes in good yields with excellent stereoselectivity. The mechanism study shows that C–F bond activation via oxidative addition is involved and PPh3 is responsible for the excellent stereoselectivity.

 Please wait while we load your content...
Please wait while we load your content...