Synthesis, and antibacterial activity of novel 4,5-dihydro-1H-pyrazole derivatives as DNA gyrase inhibitors
Abstract
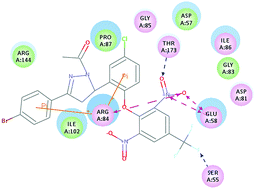
A series of novel 4,5-dihydropyrazole derivatives (4a–4t), containing the dinitrobenzotrifluoride moiety, as DNA gyrase inhibitors were designed and synthesized. Based on the preliminary results, compounds 4d, 4h and 4t with potent inhibitory activity in bacterial growth may be wonderful antibacterial agents; among them, compound 4t displayed the most potent activity with minimum inhibitory concentration (MIC) values of 3.125, 0.39, 0.39 and 0.39 μg mL−1 against Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli respectively, which was comparable with penicillin and kanamycin B with corresponding MIC values of 3.125, 3.125, 0.39, 0.39 μg mL−1 and 1.562, 1.562, 1.562, 1.562 μg mL−1, respectively. In particular, compound 4d showed the most potent anti-Gram-positive bacterial activity with a MIC value of 0.39 μg mL−1 against the tested Gram-positive bacterial strains and exhibited the most potent B. subtilis DNA gyrase and S. aureus DNA gyrase inhibitory activity with an IC50 of 0.125 μg mL−1. Docking simulation was performed to insert compound 4d into the S. aureus DNA gyrase active site to determine the probable binding conformation.

 Please wait while we load your content...
Please wait while we load your content...