Synthesis of the 2-methylene analogue of the HRV 3C protease inhibitor thysanone (2-carbathysanone)†
Abstract
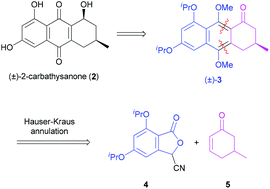
The Human Rhinovirus (HRV) is the major aetiological agent for the common cold, for which only symptomatic treatment is available. HRV maturation and replication is entirely dependent on the activity of a virally encoded 3C protease that represents an attractive target for the development of therapeutics to treat the common cold. Herein we report the synthesis and biological evaluation of the 2-methylene analogue of the HRV 3C protease inhibitor (−)-thysanone (1) namely 2-carbathysanone (2), in an attempt to decipher the structural features in the natural product that are responsible for the 3C protease activity. 2-Carbathysanone (2) (and related analogues (±)-cis-23, (±)-cis-30, (±)-31) did not inhibit HRV 3C protease, indicating that the lactol functionality present in (−)-thysanone (1) is a critical structural feature required for inhibition.

 Please wait while we load your content...
Please wait while we load your content...