Discovery of potential anti-inflammatory drugs: diaryl-1,2,4-triazoles bearing N-hydroxyurea moiety as dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase†
Abstract
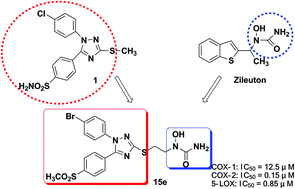
A series of hybrids from diaryl-1,2,4-triazole and hydroxamic acid or N-hydroxyurea were synthesized and evaluated as novel anti-inflammatory agents. The biological data showed that (i) all the compounds showed dual COX-2/5-LOX inhibitory activities in vitro, and 15e showed optimal inhibitory activities (COX-2: IC50 = 0.15 μM, 5-LOX: IC50 = 0.85 μM), (ii) 15e selectively inhibited COX-2 relative to COX-1 with selectivity index (SI = 0.012) comparable to celecoxib (SI = 0.015), (iii) 15e exhibited potent anti-inflammatory activity (inhibition: 54.1%) which was comparable to the reference drug celecoxib (inhibition: 46.7%) in a xylene-induced ear edema assay, and (iv) 15e displayed promising analgesic activity in acetic acid-induced writhing response and hot-plate assay. Finally, a molecular modeling study revealed the binding interactions of 15e with COX-2 and 5-LOX. Our findings suggest that 15e may be a promising anti-inflammatory agent for further evaluation.

 Please wait while we load your content...
Please wait while we load your content...