Synthesis of tetracyclic chromenones via platinum(ii) chloride catalysed cascade cyclization of enediyne–enones†
Abstract
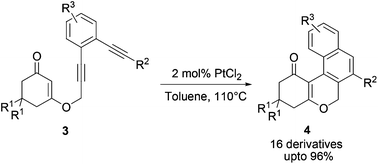
PtCl2 catalysed cascade cyclization of an enediyne–enone system to afford a tetracyclic chromenone is reported, which proceeds through two consecutive highly regioselective 6-endo–dig cyclizations in a single step with the formation of two new C–C bonds and two new rings in excellent yield. A mechanism for this transformation is proposed based on the isolated intermediates.

 Please wait while we load your content...
Please wait while we load your content...