Biocatalytic polymer thin films: optimization of the multilayered architecture towards in situ synthesis of anti-proliferative drugs†
Abstract
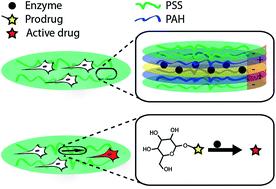
We report on the assembly of multi-layered polyelectrolyte thin films containing an immobilized enzyme to perform conversion of externally administered prodrugs and achieve delivery of the resulting therapeutics to adhering cells. Towards this goal, multi-layered coatings were assembled using poly(sodium styrene sulfonate) and poly(allylamine hydrochloride). Activity of the incorporated enzyme was quantified as a function of the assembly conditions, position of the enzyme within the multi-layered architecture, concentration of the enzyme in the adsorption solution, and concentration of the administered prodrug. Biocatalytic coatings exhibited sustained levels of enzymatic activity over at least one week of incubation in physiological buffers without signs of loss of activity of the enzyme. Developed enzyme-containing polymer films afforded zero-order release of the in situ synthesized cargo with kinetics of synthesis (nM per hour) covering at least 3 orders of magnitude. Internalization of the synthesized product by adhering cells was visualized using a fluorogenic enzyme substrate. Therapeutic utility of biocatalytic coatings was demonstrated using a myoblast cell line and a prodrug for the anti-proliferative agent, 5-fluorouridine. Taken together, this work presents a novel approach to delivery of small molecule drugs using multi-layered polymer thin films with utility in surface-mediated drug delivery, assembly of therapeutic implantable devices, and tissue engineering.

 Please wait while we load your content...
Please wait while we load your content...