High-surface-area mesoporous TiO2 microspheres via one-step nanoparticle self-assembly for enhanced lithium-ion storage†
Abstract
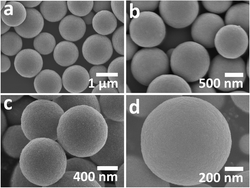
Mesoporous TiO2 microspheres assembled from TiO2 nanoparticles with specific surface areas as high as 150 m2 g−1 were synthesized via a facile one-step solvothermal reaction of titanium isopropoxide and anhydrous acetone. Aldol condensation of acetone gradually releases structural H2O, which hydrolyzes and condenses titanium isopropoxide, forming TiO2 nanocrystals. Simultaneous growth and aggregation of TiO2 nanocrystals leads to the formation of high-surface-area TiO2 microspheres under solvothermal conditions. After a low-temperature post-synthesis calcination, carbonate could be incorporated into TiO2 as a dopant with the carbon source coming from the organic byproducts during the synthesis. Carbonate doping modifies the electronic structure of TiO2 (e.g., Fermi level, Ef), and thus influences its electrochemical properties. Solid electrolyte interface (SEI) formation, which is not common for titania, could be initiated in carbonate-doped TiO2 due to elevated Ef. After removing carbonate dopants by high-temperature calcination, the mesoporous TiO2 microspheres showed much improved performance in lithium insertion and stability at various current rates, attributed to a synergistic effect of high surface area, large pore size and good anatase crystallinity.

 Please wait while we load your content...
Please wait while we load your content...