Metalization of Li2S particle surfaces in Li–S batteries†
Abstract
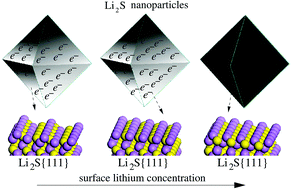
The Li–S battery is the most promising candidate for future electric vehicles. The study of stabilities and conductivities of Li2S nanoparticles, which are used as pre-lithiated cathode materials, is crucial for the development of Li–S batteries. Here, we investigate the atomic and electronic structures as well as stabilities of Li2S surfaces and nanoparticles using density functional theory (DFT) and classical electrostatic models. We show that Li2S nanoparticles have octahedral shape and consist of only (111) facets. At low concentrations of Li, the surfaces of nanoparticles are metalized non-polar Li2S(111) surfaces. The metalization is found to be due to the depletion of valence bands of surface states. However, for higher concentrations of Li, the nanoparticle faces are insulator non-polar Li2S(111) surfaces. This study suggests that Li2S nanoparticles with (111) surfaces are very promising cathode materials for Li–S batteries.

 Please wait while we load your content...
Please wait while we load your content...