Quantitative study of protein–protein interactions by quartz nanopipettes†
Abstract
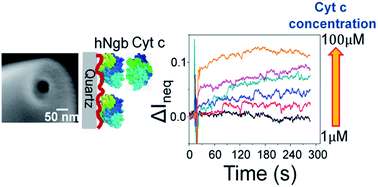
In this report, protein-modified quartz nanopipettes were used to quantitatively study protein–protein interactions in attoliter sensing volumes. As shown by numerical simulations, the ionic current through the conical-shaped nanopipette is very sensitive to the surface charge variation near the pore mouth. With the appropriate modification of negatively charged human neuroglobin (hNgb) onto the inner surface of a nanopipette, we were able to detect concentration-dependent current change when the hNgb-modified nanopipette tip was exposed to positively charged cytochrome c (Cyt c) with a series of concentrations in the bath solution. Such current change is due to the adsorption of Cyt c to the inner surface of the nanopipette through specific interactions with hNgb. In contrast, a smaller current change with weak concentration dependence was observed when Cyt c was replaced with lysozyme, which does not specifically bind to hNgb. The equilibrium dissociation constant (KD) for the Cyt c–hNgb complex formation was derived and the value matched very well with the result from surface plasmon resonance measurement. This is the first quantitative study of protein–protein interactions by a conical-shaped nanopore based on charge sensing. Our results demonstrate that nanopipettes can potentially be used as a label-free analytical tool to quantitatively characterize protein–protein interactions.

 Please wait while we load your content...
Please wait while we load your content...