Luminescent iron clusters in solution†
Abstract
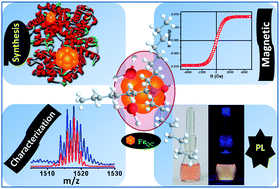
Metal clusters, composed of a few atoms at the core, exhibit unique properties and have potential applications. Although atomically precise clusters of noble metals have been synthesized, analogous systems of reactive metals, such as iron, have not been realized in solution due to high reactivity. Here we report the synthesis and characterization of novel iron clusters in the hemoglobin matrix that are highly luminescent (quantum yield 10% at 565 nm). The super-paramagnetic iron clusters, after successful ligand exchange from protein and phase transfer from water to chloroform using tri-octylphosphineoxide (TOPO), were detected as [Fe10(TOPO)3(H2O)3]+, [Fe13(TOPO)2(H2O)]+ and [Fe8(TOPO)(H2O)2]+ by mass spectrometry. This study lays the groundwork for exploiting unique properties of soluble iron clusters.

 Please wait while we load your content...
Please wait while we load your content...