Ultrafine single-crystal TiOF2 nanocubes with mesoporous structure, high activity and durability in visible light driven photocatalysis†
Abstract
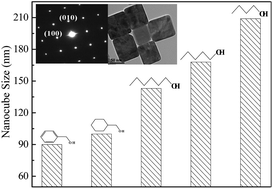
Single crystal TiOF2 nanocubes assembled into a mesoporous structure were synthesized by alcoholysis of TiF4 under solvothermal conditions, which displayed spectral response in the visible area owing to the intrinsic narrow energy band gap. Mechanism studies revealed that TiOF2 was formed via consecutive hydrolysis reactions and the H2O produced by condensation between two alcohols played a key role in determining the TiOF2 crystal growth and its transformation to anatase TiO2. The TiOF2 nanocube size could be easily adjusted by changing either alcoholysis time, or solvothermal temperature, or alcohol kind owing to the different H2O production rate and amount. The small-sized TiOF2 nanocubes with large surface area exhibited high activity in photocatalytic degradation of Rhodamine B (RhB) and 4-chlorophenol (4-CP) owing to the enhanced adsorption for reactant molecules and the reduced photoelectron–hole recombination rate. Meanwhile, they also showed strong durability since the mesoporous structure enhanced the stability against either the phase transformation from TiOF2 crystal to anatase TiO2 or the agglomeration of TiOF2 nanocubes.

 Please wait while we load your content...
Please wait while we load your content...