The vinylogous Mukaiyama aldol reaction (VMAR) in natural product synthesis
Abstract
Covering: 1973 to the end of 2013
The vinylogous Mukaiyama aldol reaction (VMAR) allows efficient access to larger segments for complex natural product synthesis, primarily polyketides, through the construction of vicinal hydroxyl and methyl groups as well as di and tri-substituted double bonds in one single operation. In this review, we will highlight stereoselective protocols that have been used in natural product synthesis and cluster them into the four groups that can be obtained from different silyl ketene acetals or enol ethers. At the beginning, an overview on different stereoselective VMARs is presented; disregarding their applications in total syntheses.
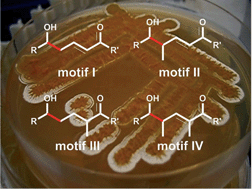
- This article is part of the themed collection: Synthesis III

 Please wait while we load your content...
Please wait while we load your content...