Conformational studies on substituted ε-caprolactams by X-ray crystallography and NMR spectroscopy†‡
Abstract
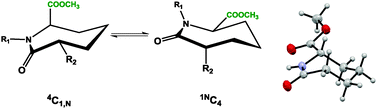
The synthesis and conformational analysis of ε-caprolactams containing a –COOMe group at the C-6 position is described. The influence of different C-2, C-6 and N substituents on ring conformation was studied using X-ray crystallography and NMR spectroscopy. The results provide evidence that all the analysed caprolactams adopt a chair type conformation with a planar lactam. In the 6-substituted caprolactam, the –COOMe residue prefers to reside in an equatorial position, but can be induced to occupy an axial orientation by the introduction of a bulky tert-butyloxycarbonyl (BOC) group on the lactam nitrogen or by C-2/C-3 ring desaturation. The BOC protected caprolactam was found to undergo exchange between two chair forms as detected by solution NMR, one with the C-6 ester equatorial (30%) and the other with it in the axial position (70%); the latter was observed by X-ray crystallography. For the C-2 dithiocarbamate substituted C-6 methyl ester seven-membered rings, a single chair form is observed for cis-isomers with both substituents equatorial. The analogous trans-isomers, however, exist as two chair forms in a 1 : 1 equilibrium ratio of 1,NC4 and 4C1,N conformers, where either substituent can occupy axial or equatorial positions.

 Please wait while we load your content...
Please wait while we load your content...