Electrode-modified with nanoparticles composed of 4,4′-bipyridine-silver coordination polymer for sensitive determination of Hg(ii), Cu(ii) and Pb(ii)†
Abstract
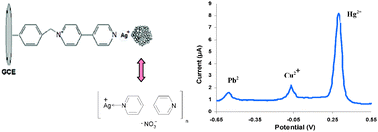
We have modified a glassy carbon electrode (GCE) with nanoparticles composed of a 4,4′-bipyridine-silver coordination polymer (CP) and showed that this CP can be applied to the sensitive differential pulse voltammetric analysis of the ions Hg(II), Cu(II) and Pb(II). The coordination polymer was prepared by mixing a solution of silver nitrate and 4,4′-bipyridine at room temperature. The surface of the GCE was modified with an organic layer of synthesized 1-[(4-nitrophenyl)methyl]-4,4′-bipyridinium and silver ions, which caused the binding of the added Ag-bipy CP. Anodic (oxidative) peaks of the electrode were at +300 mV for Hg(II), −70 mV for Cu(II), and at −540 mV for Pb(II) [versus Ag/AgCl]. Under optimal conditions, calibration graphs were linear in concentration ranges from 0.2 to 10 μg L−1 for Hg(II), from 1.3 to 6.4 μg L−1 for Cu(II), and from 4.1 to 20.7 μg L−1 for Pb(II). The respective detection limits were 0.09 μg L−1 Hg(II), 0.71 μg L−1 Cu(II) and 2.3 μg L−1 Pb(II). Relative standard deviation was 3.2% at a level of 4 μg L of Hg(II) for n = 10. The modified electrode was applied to the analysis of Hg(II) in spiked fish samples, and of Cu(II), Pb(II), and Hg(II) in spiked plant samples, and recoveries ranged from 90 to 108%. This is the first paper that presents the use of 4,4′-bipyridine-silver coordination polymer for heavy metal electrochemical detection.

 Please wait while we load your content...
Please wait while we load your content...