Synthesis, X-ray structure, spectral and electrochemical properties of a β-meso covalently linked BODIPY–Ru(ii) dipyrrin complex†
Abstract
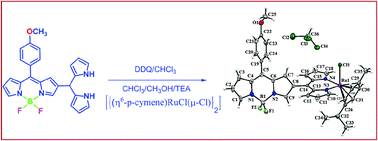
We synthesized the BODIPY–Ru(II) dipyrrin complex in decent yield by reacting β-dipyrromethanyl substituted BODIPY with [{(η6-C10H14)RuCl(μ-Cl)}2] under simple reaction conditions. The BODIPY–Ru(II) dipyrrin complex was characterized by 1D, 2D, 13C, 11B, 19F NMR, HR-MS, UV-vis, fluorescence and electrochemical techniques. The BODIPY–Ru(II) dipyrrin complex was further characterized by X-ray structure analysis, which showed that the Ru(II)-dipyrrin complex is in perpendicular orientation to the BODIPY plane in the BODIPY–Ru(II) dipyrrin complex. The ruthenium metal ion in the BODIPY–Ru(II) dipyrrin complex is bonded to dipyrrin nitrogens, one chloro group and arene ring (arene = C10H14) in an η6 manner resulting in a hexa coordination geometry around the Ru(II) ion. The absorption and steady-state fluorescence spectra of the BODIPY–Ru(II) dipyrrin complex showed a broad and hypsochromically shifted absorption and emission bands compared to that of β-dipyrromethanyl substituted BODIPY. The electrochemical studies indicated that the BODIPY–Ru(II) dipyrrin complex was easy to reduce compared to β-dipyrromethanyl substituted BODIPY supporting the electron deficient nature of the BODIPY–Ru(II) dipyrrin complex.

 Please wait while we load your content...
Please wait while we load your content...