A multi-pronged mechanistic study of the phosphine-mediated conjugate addition of an alcohol to an acetylenic ester†
Abstract
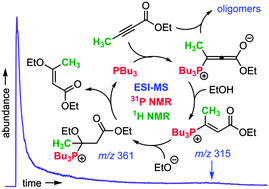
The conjugate addition of an alcohol to a butynoate ester using an organophosphine catalyst was monitored using pressurized sample infusion electrospray ionization mass spectrometry (PSI-ESI-MS), together with 31P and 1H NMR spectroscopy. The combination of methods allowed examination of the reaction progress from the perspective of reactants and products (1H NMR) and insights into behaviors of the reaction intermediates and formation of byproducts (31P NMR and MS). The resulting traces could be closely approximated through numerical modeling by appropriate selection of rate constants, and a sound understanding of the mechanism and the means by which oligomeric byproducts are formed allows a rational approach to experimental design.

 Please wait while we load your content...
Please wait while we load your content...