A kinetic analysis of CO release from a diiron hexacarbonyl complex promoted by amino acids†
Abstract
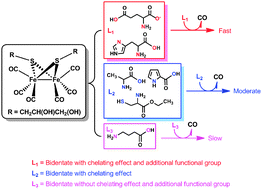
A water-soluble diiron hexacarbonyl complex, [Fe2{μ-SCH2CH(OH)CH2(OH)}(CO)6] (1), was employed as a carbon monoxide releasing molecule (CO-RM). The CO release was initiated via substitution of the bound CO by amino acids. The kinetics of the decomposition of complex 1 was a first-order process with respect to both the complex and amino acids. Its rate of CO release varies with the amino acids. Six amino acids were examined – L-cysteine ethyl ester hydrochloride, alanine, γ-aminobutyric acid, L-histidine, L-proline and sodium glutamate – as CO release promoters. Among the examined promoters, sodium glutamate shows the highest efficiency in promoting CO release from complex 1. The overall CO release involves multiple steps with different mechanisms, with an initially slow CO releasing process followed by a much faster one. The duration of the slow process is solvent-dependant. In D2O and physiological saline (in D2O), this slow process lasted much longer compared to that in DMSO. Cytotoxic assessments of the systems containing glutamate and L-cysteine ethyl ester hydrochloride suggest that the examined amino acid and amino acid derivative showed stronger cytotoxicities than cysteamine.

 Please wait while we load your content...
Please wait while we load your content...