Hexahydrophthalimide–benzothiazole hybrids as a new class of protoporphyrinogen oxidase inhibitors: synthesis, structure–activity relationship, and DFT calculations
Abstract
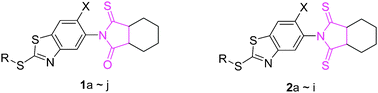
Protoporphyrinogen oxidase (PPO, EC 1.3.3.4) has attracted continuous interest during the last few decades not only because of its unique biochemical characteristics but also because of its biomedical significance. As a continuation of our research work on the development of new PPO inhibitors, N-(benzothiazol-5-yl)-hexahydro-2H-isoindole-1,3-dithione (1a–j) and N-(benzothiazol-5-yl)-octahydro-3-thioxoisoindol-1-one derivatives (2a–i) were designed and synthesized. These newly prepared compounds were characterized by elemental analyses, 1H NMR and ESI-MS spectroscopy. The in vitro assay indicated that these compounds displayed good inhibition activity against human PPO (hPPO) with Ki values ranging from 0.38 μM to 6.83 μM. Notably, most of the monothionated products (1a–j) displayed a higher or comparable PPO-inhibition activity compared with the commercial control sulfentrazone. The comparison of the dihedral angles of the representative compound with that of acifluorfen (ACF) complexed with hPPO clearly indicated that the dihedral angle between the thionyl amide or carbonyl amide ring and the benzothiazole ring was closely related to the variation of the PPO inhibition activity of different types of inhibitors.

 Please wait while we load your content...
Please wait while we load your content...