Multinuclear ruthenium(ii) complexes as anticancer agents
Abstract
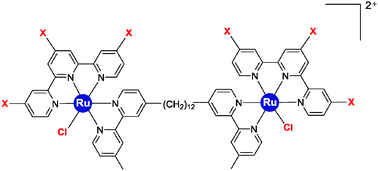
A series of dinuclear ruthenium(II) complexes that contain labile chlorido ligands, [{Ru(tpy)Cl}2{μ-bbn}]2+ {designated Cl-Rubbn; tpy = 2,2′:6′,2′′-terpyridine, bbn = bis[4(4′-methyl-2,2′-bipyridyl)]-1,n-alkane (n = 7, 10, 12, 14 or 16)} and derivatives containing nitro substituents on the tpy ligand and/or secondary amines within the bbn linking chain have been synthesised and their potential as anticancer agents examined. Some of the Cl-Rubbn species showed good anticancer activity against MCF-7 and MDA-MB-231 breast cancer cell lines, with the Cl-Rubb12 complex being four-times more active than cisplatin. Inclusion of nitro substituents on the tpy ligands of Cl-Rubb12 resulted in significantly decreased anticancer activity. The incorporation of amine groups into the linking ligand did not increase the anticancer activity of the Cl-Rubbn complexes. The Cl-Rubbn complexes and those containing amine groups in the linking chain aquated at approximately the same rate, with 50% aquation within 120 minutes. By comparison, the complexes containing nitro substituents on the tpy ligand aquated extremely slowly, with 60% of the chlorido complex remaining 24 hours after they were dissolved in water. Cyclic voltammetry with the model mononuclear complex [Ru{(NO2)3tpy}(Me2bpy)Cl]+ {(NO2)3tpy = 4,4′,4′′-trinitro-2,2′:6′,2′′-terpyridine} showed that the nitro substituents exerted a strong effect on the ruthenium centre, with the anodic peak corresponding to the Ru(III/II) couple shifted positively by 300 mV compared to that from the non-nitrated parent complex [Ru(tpy)(Me2bpy)Cl]+. 1H NMR studies of the reaction of the Cl-Rubbn complexes with GMP indicated that the ruthenium complexes covalently bound the nucleotide slowly, with 33% bound in 24 hours. However, the results of this study suggest that the cytotoxicity of the dinuclear ruthenium complexes is a combination of covalent and reversible binding with DNA.

 Please wait while we load your content...
Please wait while we load your content...