From spiropentane to butterfly and tetrahedral structures in tetranuclear iron carbonyl carbide chemistry†
Abstract
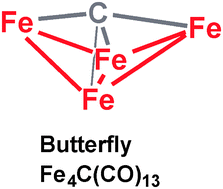
Oxidative degradation of the octahedral dianion [Fe6C(CO)16]2− with an interstitial carbon atom leads eventually to the neutral Fe4C(CO)13 cluster with a butterfly-shaped central Fe4C unit. The complete series of related Fe4C(CO)n (n = 16, 15, 14, 13, 12, 11) derivatives have now been investigated using density functional theory. For the lowest energy Fe4C(CO)n (n = 16, 15, 14, 13) structures the geometries obey the n + f = 18 rule where f is the number of Fe–Fe bonds. This leads to a spiropentane geometry with two Fe–Fe bonds for Fe4C(CO)16, a central bent Fe–Fe–Fe–Fe chain for Fe4C(CO)15, a distorted trigonal pyramidal structure with four Fe–Fe bonds for Fe4C(CO)14, and the experimentally observed butterfly structure with five Fe–Fe bonds for Fe4C(CO)13. A symmetrical higher energy centered tetrahedral structure for Fe4C(CO)12 with six Fe–Fe bonds also follows the n + f = 18 rule. However, the lowest energy Fe4C(CO)n (n = 12, 11) structures are derived from the lowest energy Fe4C(CO)13 structure by removal of CO groups with retention of the central Fe4C butterfly unit.

 Please wait while we load your content...
Please wait while we load your content...