Chitosan: an efficient promoter for the synthesis of 2-aminopyrimidine-5-carbonitrile derivatives in solvent free conditions
Abstract
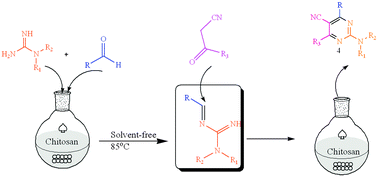
Reusable chitosan without any post-modification, with active –NH2 and –OH groups, was found to be a highly efficient and renewable heterogeneous catalyst for the rapid and convenient synthesis of 2-aminopyrimidine-5-carbonitrile derivatives from guanidines, aldehydes and cyanoketones, under mild reaction conditions at 85 °C in good yields. Undoubtedly this methodology gives a facile and straightforward pathway to construct 2-aminopyrimidine-5-carbonitrile derivatives in an eco-friendly fashion.

 Please wait while we load your content...
Please wait while we load your content...