N-Heterocyclic carbene rhodium(i) complexes containing an axis of chirality: dynamics and catalysis†
Abstract
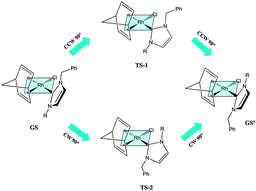
The novel rhodium(I) complexes [RhCl(NBD)(NHC)] [NBD = norbornadiene, NHC = 1-benzyl-3-R-imidazolin-2-ylidene; R = Me (3a), Bz (3b), Tr (3c), tBu (3d)], containing on one nitrogen the benzyl substituent and on the other increasing bulky alkyl substituents were prepared. All the complexes display restricted rotation around the metal–carbene bond and yield conformational enantiomers. The stereodynamics and racemization barriers about the Rh–carbene have been determined by means of NMR spectroscopy for 3a–c, whereas for the bulkiest 3d only the lower limit (91 kJ mol−1) could be calculated. Whilst the racemization barriers obtained by DFT calculations for 3a,b and 3d matched the experimental values, in the case of 3c the latter (62.3 kJ mol−1) was much smaller with respect to the calculated one (101.7 kJ mol−1). The lower experimental barrier has been attributed to a dissociative pathway that produces a solvated ionic pair in the transition state. The catalytic activity of the neutral rhodium(I) complexes 3a and 3d in the hydrosilylation with HSiMe2Ph of the terminal alkynes PhC≡CH, TolC≡CH, nBuC≡CH, Et3SiC≡CH, and (CPh2OH)C≡CH has been investigated, and compared with the amide-functionalized [RhCl(NBD){1-(2-NHBoc-ethyl)-3-Me-imidazolin-2-ylidene}] (4) and with [RhCl(NBD){1-butyl-3-Me-imidazolin-2-ylidene}] (5).

 Please wait while we load your content...
Please wait while we load your content...