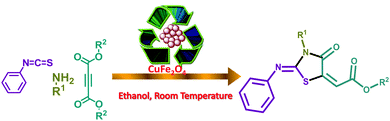
A facile and efficient synthesis of functionalized 4-oxo-2-(phenylimino)thiazolidin-5-ylideneacetate derivatives via a CuFe2O4 magnetic nanoparticles catalyzed regioselective pathway†
Abstract
Nano-CuFe2O4 spinel has been found to exhibit strong catalytic activity in the cascade reaction involving 1,4-addition and intramolecular electrophilic cyclization in a perfectly regiocontrolled manner and a series of functionalized 4-oxo-2-(phenylimino)thiazolidin-5-ylideneacetate derivatives were generated successfully. The reaction is mild and selective with good to high yields and displays significant functional group tolerance. The dual role of the thiocarbonyl moiety as an electrophile and as a nucleophile has been observed in this three component coupling reaction. CuFe2O4 magnetic nanoparticles were prepared by a simple and effective citric acid complex method and characterized by using XRD, FT-IR, and TEM analysis. The catalyst was recycled for six cycles without any loss of catalytic activity. All reactions were easily performed and proceeded with high efficiency under very simple and mild conditions and gave excellent yields avoiding time-consuming, costly syntheses, tedious workup and purification hazards.

 Please wait while we load your content...
Please wait while we load your content...