Two novel aggregation-induced emission active coumarin-based Schiff bases and their applications in cell imaging†
Abstract
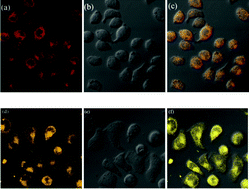
Two new coumarin-based Schiff bases, 8,8′-((1E,1′E)-hydrazine-1,2-diylidenebis(methanylylidene))bis(7-hydroxy-4-methyl-2H-chromen-2-one), (CHC), and 7-hydroxy-8-((E)-((E)-((2-hydroxynaphthalen-1-yl)methylene)hydrazono)methyl)-4-methyl-2H-chromen-2-one, (CHN), with excited-state intramolecular proton-transfer (ESIPT) properties, were synthesized and characterized. Both of the compounds displayed aggregation-induced emission (AIE) characteristics, of which CHC nanoparticles emitted a reddish orange fluorescence, while the CHN nanoparticles exhibited a saffron yellow fluorescence. The appearance of emission peaks in the long wave regions with large Stokes-shifts demonstrated the occurrence of the ESIPT process. Observations of the nanoparticles' morphologies were undertaken through a transmission electron microscope (TEM) method. Furthermore, due to the good biocompatibilities, high stabilities and the large Stokes-shifts, the two compounds were ideal candidates for cell staining.

 Please wait while we load your content...
Please wait while we load your content...