Efficient C-3 reductive alkylation of 4-hydroxycoumarin by dehydrogenative oxidation of benzylic alcohols through ruthenium catalysis†
Abstract
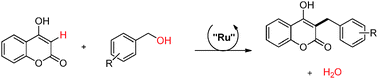
A practical route to prepare 4-hydroxycoumarin derivatives functionalized at the C-3 position is described through a catalytic coupling reaction between 4-hydroxycoumarin and a series of substituted benzylic alcohols. The reaction is conducted in the presence of tris(triphenylphosphine)ruthenium(II) dichloride (5 mol%), KOH (0.2 eq.) in tertamyl alcohol under microwave irradiation at 140 °C for 2 hours.

 Please wait while we load your content...
Please wait while we load your content...