An in situ spectroscopic study on decomposition of MgSiO3 during the alkali fusion process using sodium hydroxide
Abstract
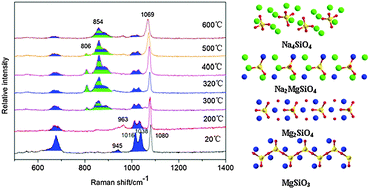
The mechanism of decomposition of magnesium inosilicate (MgSiO3) during the alkali fusion process using NaOH was investigated by Raman spectroscopy in situ and X-ray diffraction analyses. The results show that the tetrahedral silica chains within MgSiO3 are gradually disrupted, and nesosilicate with the isolated SiO4 tetrahedra becomes reorganized at the beginning of the alkali fusion process. In the decomposition of MgSiO3, the two intermediates are Mg2SiO4 and Na2MgSiO4, while the final products are Mg(OH)2 and Na4SiO4. It can be concluded that this decomposition did not initiate from the cation exchange reaction in the process.

 Please wait while we load your content...
Please wait while we load your content...