Galvanic replacement of As(0) nanoparticles by Au(iii) for nanogold fabrication and SERS application†
Abstract
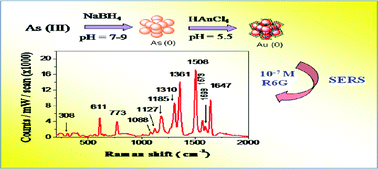
A galvanic replacement reaction between As(0) nanoparticles and Au(III) ions has been reported for the first time. Initially the stable yellow-brown As(0) nanoparticles were prepared by the borohydride reduction of an arsenite solution. The characterization of the As(0) particles was discussed in a recent report. In the present work these As(0) nanoparticles were exploited to fabricate gold nanoparticles (AuNPs). The as-obtained red colored gold sol showed a λmax at 540 nm and the size of the AuNPs were 62 ± 7 nm as observed from TEM analyses. It was interesting to note that the size of the AuNPs was comparable to that of the As(0) nanoparticles, which could be a sign of galvanic replacement in the absence of any stabilizer. The particles were spherical with a hollow core. The AuNPs were characterized by SEM, TEM, XRD, DLS and UV-visible spectroscopy. FTIR and Raman analysis indicated that during the galvanic replacement reaction As(0) was oxidized to arsenate, which stabilized the AuNPs through adsorption and H-bonding. Thus a stable assembly of AuNPs was obtained in the absence of any external stabilizer. The potential of such an assembly was further exploited for SERS detection of Rhodamine 6G, 4-mercaptopyridine and 4-aminothiophenol.

 Please wait while we load your content...
Please wait while we load your content...