Metal triangles versus metal chains and terminal versus bridging hydrogen atoms in trinuclear osmium carbonyl hydride chemistry†
Abstract
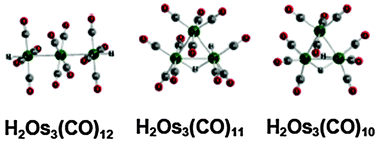
The chemistry of trinuclear osmium carbonyl hydrides is a rich area with the three H2Os3(CO)n derivatives (n = 12, 11, 10) all being known stable compounds ultimately obtained from Os3(CO)12 and hydrogen under various conditions. Density functional theory studies on the H2Os3(CO)n systems (n = 12, 11, 10, 9, 8) correctly predict the structures previously reported experimentally for n = 12, 11, and 10. These include a linear structure for H2Os3(CO)12 and triangular structures for H2Os3(CO)11 and H2Os3(CO)10. However, the H2Os3(CO)11 system is predicted to be a fluxional system with the four low energy isomers lying within 2 kcal mol−1 of energy. Three of these H2Os3(CO)11 isomers, all with one terminal hydrogen and one bridging hydrogen, have been observed experimentally by NMR. In addition, the lowest energy isomer has been isolated and structurally characterized by X-ray crystallography. In contrast to H2Os3(CO)11, the lowest energy H2Os3(CO)10 structure, namely the known structure with an Os![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Os edge bridged by both hydrogen atoms and all terminal CO groups, lies ∼10 kcal mol−1 below the next lowest energy isomer. The predicted CO dissociation energies of the H2Os3(CO)n derivatives (n = 12, 11, 10) suggest this H2Os3(CO)10 structure to be the “thermodynamic sink” in the H2Os3(CO)n systems, consistent with its synthesis from Os3(CO)12 and H2 at 120 °C and atmospheric pressure. The lowest energy structures of the more highly unsaturated H2Os3(CO)n (n = 9, 8) can be derived from this (μ-H)2Os3(CO)10 structure by removal of CO groups from the osmium atom remote to the doubly bridged Os
Os edge bridged by both hydrogen atoms and all terminal CO groups, lies ∼10 kcal mol−1 below the next lowest energy isomer. The predicted CO dissociation energies of the H2Os3(CO)n derivatives (n = 12, 11, 10) suggest this H2Os3(CO)10 structure to be the “thermodynamic sink” in the H2Os3(CO)n systems, consistent with its synthesis from Os3(CO)12 and H2 at 120 °C and atmospheric pressure. The lowest energy structures of the more highly unsaturated H2Os3(CO)n (n = 9, 8) can be derived from this (μ-H)2Os3(CO)10 structure by removal of CO groups from the osmium atom remote to the doubly bridged Os![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Os edge of the Os3 triangle, with relatively little change in the central (μ-H)2Os3 triangle geometry.
Os edge of the Os3 triangle, with relatively little change in the central (μ-H)2Os3 triangle geometry.

 Please wait while we load your content...
Please wait while we load your content...