Synthesis and fluorescence properties of isoindoline–benzazole-based boron difluoride complexes†
Abstract
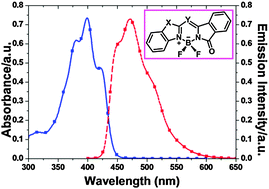
In this article, a series of isoindoline–benzazole-based boron difluoride complexes (7–12) were synthesized and characterized. Analysis of the X-ray crystal structures of compounds 7, 8 and 10–12 indicates the existence of π–π stacking and H-bond (F–H–C, O–H–N, N–H–C) interactions. These novel boron complexes exhibit large Stokes shifts (3300–8400 cm−1) and moderate quantum yields (0.15–0.64). Time-dependent DFT (TD-DFT) calculations reveal that the maximum absorption bands for 7–12 are attributed to the HOMO → LUMO transitions, and that the calculated absorption wavelengths agree well with the experimental trends.

 Please wait while we load your content...
Please wait while we load your content...