Kinetic studies of hydroxyquinone formation from water soluble benzoquinones†
Abstract
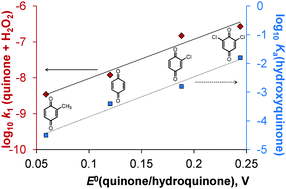
The kinetics and mechanisms of the redox reactions between hydrogen peroxide and 1,4-benzoquinone, 2-methyl-1,4-benzoquinone, 2,6-dimethyl-1,4-benzoquinone, 2-chloro-1,4-benzoquinone and 2,6-dichloro-1,4-benzoquinone were studied in aqueous media using spectrophotometric monitoring. The formation and decay of a hydroxylated 1,4-benzoquinone was detected. The formation of the intermediate was first order with respect to the parent 1,4-benzoquinone and hydrogen peroxide, whereas inverse first order dependence was revealed with respect to the hydrogen ion. The decomposition reaction had two parallel pathways: one was first order with respect to the intermediate, while the other showed second-order dependence. The values of the rate constant measured for the formation step were successfully correlated with both the redox potentials of the substituted quinone–hydroquinone systems and the pKa values of the hydroxylated quinone derivatives. Therefore, electronic effects govern the reactivity of the quinones in this process. NMR and GC-MS measurements were carried out to identify the products in the system. Quantum mechanical calculations were also carried out in these systems.

 Please wait while we load your content...
Please wait while we load your content...