Color stability and spectroscopic properties of deoxyvitisins in aqueous solution†
Abstract
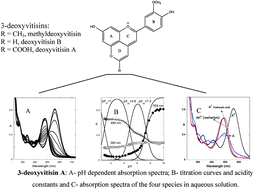
The color properties and stability of three types of 3-deoxyvitisins derived from peonidin (deoxyvitisin A, B and methyldeoxyvitisin) were studied using UV-Visible spectroscopy. Similarly to pyranoanthocyanins, the conjugated double bonds among pyranic rings C and D provide a higher electronic delocalization that prevents the nucleophilic attack of water at position 2 and the subsequent formation of the hemiketal and chalcone species. Consequently, besides the flavylium cation (AH+), neutral (A) and ionized bases (An−) have been identified by increasing pH, and the respective acidity constants were determined. The acidity constant values for the formation of the neutral quinoidal base (pKa2(deoxyvit A) = 4.8, pKa1(deoxyvit B) = 4.7 and pKa1(methyldeoxyvitisin) = 5.2) are slightly higher than the ones reported in the literature for their corresponding 3-glucosyl derivatives (vitisins). Given their higher stability, these pigments may be regarded as potential food colorants.

 Please wait while we load your content...
Please wait while we load your content...