A new polymeric complex of silver(i) with a hybrid pyrazine–bipyridine ligand – synthesis, crystal structure and its photocatalytic activity†
Abstract
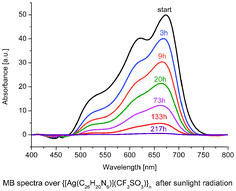
The 2,3-bis(6′-methyl-2,2′-bipyridin-6-yl)pyrazine ligand L reacts with trifluoromethanesulfonate silver(I) to give a coordination polymer {[AgL](CF3SO3)}n in which metal ions are in a distorted tetragonal pyramidal coordination geometry. The complex has been characterized by spectroscopic techniques, elemental analysis, X-ray diffraction, and UV-Vis spectroscopy. The methylene blue (MB) degradation was studied using UV-Vis spectrophotometry. After 400 min of exposure to UV light MB was completely decomposed. Degradation of MB after exposure to sunlight was considerably slower: 34% after 400 min and 90% after 133 h. Photodegradation of the dye follows second-order kinetics. {[AgL](CF3SO3)}n is an active photocatalyst for MB degradation under UV-Vis and sunlight irradiation.

 Please wait while we load your content...
Please wait while we load your content...