Strengthening of hydrogen bonded coumarin 102 in ethanol solvent upon photoexcitation
Abstract
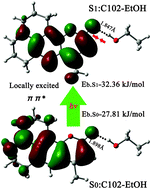
Excited state hydrogen-bonding dynamics of coumarin in ethanol solvent has been studied using TDDFT/6-311++G(d,p) and a conductor-like polarizable continuum model. The geometry, hydrogen bond binding energy and frequency analysis indicate that the intermolecular hydrogen bond between C102 and ethanol is strengthened in the excited state. The binding energy is increased from 27.81 kJ mol−1 in the ground state to 32.36 kJ mol−1 in the S1 state. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–H stretching bands in C102–EtOH are strongly red-shifted due to the formation of the intermolecular hydrogen bond. In the excited state, redshift occurs for the C
O and O–H stretching bands in C102–EtOH are strongly red-shifted due to the formation of the intermolecular hydrogen bond. In the excited state, redshift occurs for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–H in the C102–EtOH complex. The excitation energy and frontier molecular orbital analysis indicate that S1 of the C102–EtOH complex is the locally excited state. S1 corresponds to the orbital transition from the HOMO to the LUMO with the ππ* character. Furthermore, the internal conversion from the excited to ground state is enhanced by hydrogen bond strengthening.
O and O–H in the C102–EtOH complex. The excitation energy and frontier molecular orbital analysis indicate that S1 of the C102–EtOH complex is the locally excited state. S1 corresponds to the orbital transition from the HOMO to the LUMO with the ππ* character. Furthermore, the internal conversion from the excited to ground state is enhanced by hydrogen bond strengthening.

 Please wait while we load your content...
Please wait while we load your content...