Zn-bis-glutathionate is the best co-substrate of the monomeric phytochelatin synthase from the photosynthetic heavy metal-hyperaccumulator Euglena gracilis†
Abstract
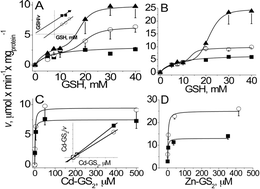
The phytochelatin synthase from photosynthetic Euglena gracilis (EgPCS) was analyzed at the transcriptional, kinetic, functional, and phylogenetic levels. Recombinant EgPCS was a monomeric enzyme able to synthesize, in the presence of Zn2+ or Cd2+, phytochelatin2–phytochelatin4 (PC2–PC4) using GSH or S-methyl-GS (S-methyl-glutathione), but not γ-glutamylcysteine or PC2 as a substrate. Kinetic analysis of EgPCS firmly established a two-substrate reaction mechanism for PC2 synthesis with Km values of 14–22 mM for GSH and 1.6–2.5 μM for metal-bis-glutathionate (Me-GS2). EgPCS showed the highest Vmax and catalytic efficiency with Zn-(GS)2, and was inactivated by peroxides. The EgPCS N-terminal domain showed high similarity to that of other PCSases, in which the typical catalytic core (Cys-70, His-179 and Asp-197) was identified. In contrast, the C-terminal domain showed no similarity to other PCSases. An EgPCS mutant comprising only the N-terminal 235 amino acid residues was inactive, suggesting that the C-terminal domain is essential for activity/stability. EgPCS transcription in Euglena cells was not modified by Cd2+, whereas its heterologous expression in ycf-1 yeast cells provided resistance to Cd2+ stress. Phylogenetic analysis of the N-terminal domain showed that EgPCS is distant from plants and other photosynthetic organisms, suggesting that it evolved independently. Although EgPCS showed typical features of PCSases (constitutive expression; conserved N-terminal domain; kinetic mechanism), it also exhibited distinct characteristics such as preference for Zn-(GS)2 over Cd-(GS)2 as a co-substrate, a monomeric structure, and ability to solely synthesize short-chain PCs, which may be involved in conferring enhanced heavy-metal resistance.

 Please wait while we load your content...
Please wait while we load your content...