Metallothionein-like peptides involved in sequestration of Zn in the Zn-accumulating ectomycorrhizal fungus Russula atropurpurea†
Abstract
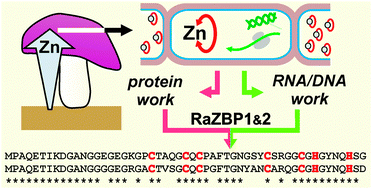
Homeostatic mechanisms preventing the toxicity of free Zn ions in cells involve, among others, cytosolic Zn-binding ligands, particularly the cysteine-rich metallothioneins (MTs). Here we examined the Zn-binding peptides of Russula atropurpurea, an ectomycorrhizal fungus known for its ability to accumulate high amounts of Zn in its sporocarps. The Zn complexes and their peptide ligands were characterized using chromatography, electrophoresis after fluorescent labeling of cysteine residues, and tandem mass spectrometry. Functional complementation assays in Saccharomyces cerevisiae were used to obtain and characterize cDNA sequences. Zn-speciation analysis showed that nearly 80% of the Zn extracted from the sporocarps was associated with cysteine-containing peptides in a 5 kDa complex. Screening of an R. atropurpurea cDNA library for sequences encoding peptides capable of sequestering divalent heavy metals was conducted in the Cd-hypersensitive ycf1Δ yeast. This allowed identification of two cDNAs, RaZBP1 and RaZBP2, which protected the metal-sensitive yeast mutants against Cd and Zn, but not Co, Mn or Cu, toxicity. The corresponding RaZBP1 and RaZBP2 peptides consisting of 53 amino acid (AA) residues and sharing 77% identity showed only a limited sequence similarity to known MTs, particularly due to the absence of multiple Cys-AA-Cys motifs. Both RaZBPs were detected in a native Zn-complex of R. atropurpurea and the recombinant RaZBP1 was found associated with Zn and Cd in yeasts. Altogether, the results point to an important role of RaZBPs in the handling of a substantial portion of the Zn pool in R. atropurpurea.

 Please wait while we load your content...
Please wait while we load your content...