Neuroprotective peptide–macrocycle conjugates reveal complex structure–activity relationships in their interactions with amyloid β†
Abstract
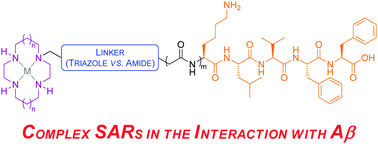
Interactions between amyloid β (Aβ) and metal ions are thought to mediate the neuropathogenic effects of Aβ in Alzheimer's disease. The construction of small molecules capable of synergistically chelating metal ions and recognizing Aβ would allow new insights into the biology of this disease and provide a possible therapeutic approach. We report herein the synthesis and biological evaluation of tetraazamacrocycle–(G)KLVFF hybrids and their metal complexes. The results obtained from ThT and bis-ANS extrinsic fluorescence assays, tyrosine intrinsic fluorescence assay and proteolytic assay imply complex, multifaceted structure–activity relationships in the interaction of these conjugates with Aβ. Many of the compounds tested rescued cells from Aβ-induced cytotoxicity. The attendant simplicity and ready diversification of the synthesis of these conjugates makes them attractive for further investigation.


 Please wait while we load your content...
Please wait while we load your content...