Redox sulfur chemistry of the copper chaperone Atox1 is regulated by the enzyme glutaredoxin 1, the reduction potential of the glutathione couple GSSG/2GSH and the availability of Cu(i)†
Abstract
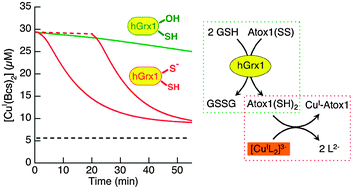
Glutaredoxins have been characterised as enzymes regulating the redox status of protein thiols via cofactors GSSG/GSH. However, such a function has not been demonstrated with physiologically relevant protein substrates in in vitro experiments. Their active sites frequently feature a Cys–xx–Cys motif that is predicted not to bind metal ions. Such motifs are also present in copper-transporting proteins such as Atox1, a human cytosolic copper metallo-chaperone. In this work, we present the first demonstration that: (i) human glutaredoxin 1 (hGrx1) efficiently catalyses interchange of the dithiol and disulfide forms of the Cys12–xx–Cys15 fragment in Atox1 but does not act upon the isolated single residue Cys41; (ii) the direction of catalysis is regulated by the GSSG/2GSH ratio and the availability of Cu(I); (iii) the active site Cys23–xx–Cys26 in hGrx1 can bind Cu(I) tightly with femtomolar affinity (KD = 10−15.5 M) and possesses a reduction potential of Eo′ = −118 mV at pH 7.0. In contrast, the Cys12–xx–Cys15 motif in Atox1 has a higher affinity for Cu(I) (KD = 10−17.4 M) and a more negative potential (Eo′ = −188 mV). These differences may be attributed primarily to the very low pKa of Cys23 in hGrx1 and allow rationalisation of conclusion (ii) above: hGrx1 may catalyse the oxidation of Atox1(dithiol) by GSSG, but not the complementary reduction of the oxidised Atox1(disulfide) by GSH unless Cuaq+ is present at a concentration that allows binding of Cu(I) to reduced Atox1 but not to hGrx1. In fact, in the latter case, the catalytic preferences are reversed. Both Cys residues in the active site of hGrx1 are essential for the high affinity Cu(I) binding but the single Cys23 residue only is required for the redox catalytic function. The molecular properties of both Atox1 and hGrx1 are consistent with a correlation between copper homeostasis and redox sulfur chemistry, as suggested by recent cell experiments. These proteins appear to have evolved the features necessary to fill multiple roles in redox regulation, Cu(I) buffering and Cu(I) transport.

 Please wait while we load your content...
Please wait while we load your content...