An integrated study of the affinities of the Aβ16 peptide for Cu(i) and Cu(ii): implications for the catalytic production of reactive oxygen species†
Abstract
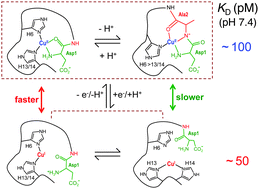
A new fluorescent probe Aβ16wwa based upon the Aβ16 peptide has been developed with two orders of magnitude greater fluorescence intensity for sensitive detection of interactions with Cu(II). In combination with the Cu(I) probe Ferene S, it is confirmed that the Aβ16 peptide binds either Cu(I) or Cu(II) with comparable affinities at pH 7.4 (log KID = −10.4; log KIID = −10.0). It follows from this property that the Cu–Aβ16 complex is a robust if slow catalyst for the aerial oxidation of ascorbate with H2O2 as primary product (initial rate, ∼0.63 min−1 for Cu–Aβ16 versus >2.5 min−1 for Cuaq2+). An integrated study of variants of this peptide identifies the major ligands and binding modes involved in its copper complexes in solution. The dependence of KID upon pH is consistent with a two-coordinate Cu(I) site in which dynamic processes exchange Cu(I) between the three available pairs of imidazole sidechains provided by His6, His13 and His14. The N-terminal amine is not involved in Cu(I) binding but is a key ligand for Cu(II). Acetylation of the N-terminus alters the redox thermodynamic gradient for the Cu centre and suppresses its catalytic activity considerably. The data indicate the presence of dynamic processes that exchange Cu(II) between the three His ligands and backbone amide at physiological pH. His6 is identified as a key ligand for catalysis as its presence minimises the pre-organisation energy required for interchange of the two copper redox sites. These new thermodynamic data strengthen structural interpretations for the Cu–Aβ complexes and provide valuable insights into the molecular mechanism by which copper chemistry may induce oxidative stress in Alzheimer's disease.

 Please wait while we load your content...
Please wait while we load your content...