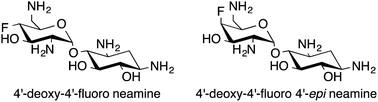
Synthesis of 4′-deoxy-4′-fluoro neamine and 4′-deoxy-4′-fluoro 4′-epi neamine†
Abstract
The syntheses of 4′-deoxy-4′-fluoro neamine and 4′-deoxy-4′-fluoro 4′-epi neamine from the readily available neamine and paromamine are described. Different approaches for the introduction of fluorine such as the use of DAST, sulfonic esters displacement, and epoxide ring opening by fluoride anion were studied. Examples of anchimeric assistance and ring contraction in the aminoglycoside series are highlighted.

 Please wait while we load your content...
Please wait while we load your content...