Fragment growing to retain or alter the selectivity of anchored kinase hinge-binding fragments†
Abstract
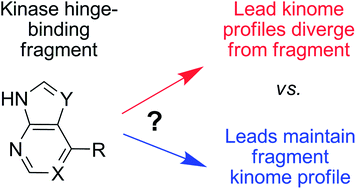
The activity patterns of kinase hinge-binding fragments can be retained or redirected in fragment growing strategies. Targeting conserved kinase features preserved the selectivity pattern of a PKB hinge-binding fragment over a 5000-fold increase in potency, while late-stage modification of a CHK1 hinge-binding fragment substantially changed the pattern.

 Please wait while we load your content...
Please wait while we load your content...