Pyrido- and benzisothiazolones as inhibitors of histone acetyltransferases (HATs)†
Abstract
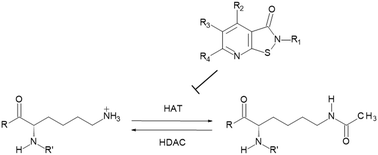
Histone acetyltransferases (HATs) are interesting targets for the treatment of cancer and HIV infections but reports on selective inhibitors are very limited. Here we report structure–activity studies of pyrido- and benzisothiazolones in the in vitro inhibition of histone acetyltransferases, namely PCAF, CBP, Gcn5 and p300 using a heterogeneous assay with antibody mediated quantitation of the acetylation of a peptidic substrate. Dependent on the chemical structure distinct subtype selectivity profiles can be obtained. While N-aryl derivatives usually are rather pan-HAT inhibitors, N-alkyl derivatives show mostly a preference for CBP/p300. Selected compounds were also shown to be inhibitors of MOF. The best inhibitors show submicromolar inhibition of CBP. Selected compounds affect growth of HL-60 leukemic cells and LNCaP prostate carcinoma cells with higher potency on the leukemic cells. Target engagement was shown with reduction of histone acetylation in LNCaP cells.

 Please wait while we load your content...
Please wait while we load your content...