A double effect molecular switch leads to a novel potent negative allosteric modulator of metabotropic glutamate receptor 5†
Abstract
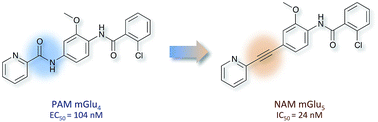
Compounds that modulate the function of G-protein-coupled receptors (GPCRs) by binding to their allosteric sites are of potential interest for the treatment of multiple CNS and non-CNS disorders. Allosteric ligands can act either as positive (PAM), negative (NAM), or silent (SAM) receptor modulators and have numerous advantages over classic orthosteric compounds, including improved GPCR-subtype selectivity; the capacity to adapt to physiological conditions; and better safety profiles. Despite these benefits, allosteric modulators are difficult to design and optimize and are often prone to “molecular switching”: a structural phenomenon by which very subtle chemical variations in the ligand result in unexpected changes in selectivity profiles or pharmacology, changing PAMs to NAMs or vice versa. Here, we report the discovery of a nanomolar and subtype selective NAM of metabotropic glutamate receptor 5 (mGlu5) through a targeted “double effect molecular switch” of a potent mGlu4 PAM, and suggests a promising approach towards the discovery of novel mGluR allosteric modulators.

 Please wait while we load your content...
Please wait while we load your content...