Synthesis of a novel series of 2,3,4-trisubstituted oxazolidines designed by isosteric replacement or rigidification of the structure and cytotoxic evaluation†
Abstract
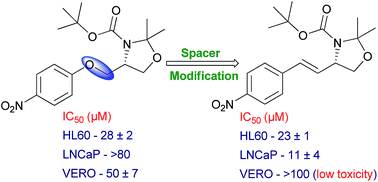
We have previously reported on a study of the structure–activity relationship in a series of 2,3,4-substituted oxazolidines recently discovered by our group varying the substituent at the ring or stereochemistry of the oxazolidine ring. We discovered the cytotoxic and pro-apoptotic potential of compounds 1 and 2 with good selectivity against cancer cell lines. In the present study we describe the synthesis and cytotoxic evaluation against cancer cell lines (HL60, JURKAT, MDA-MB-231 and LNCaP) of a series of oxazolidines designed by isosteric replacement or rigidification of the oxymethylene spacer of compounds 1 and 2. Alkenes 3 and 4 retained the activity against MDA-MB-231 cells and they were more active on HL60, JURKAT and LNCaP cells. Considering LNCaP cells, E-isomer 4 was at least 7 times and about 3 times more potent than lead 1 and Z-isomer 3, respectively. Compound 4 exerted significant activity against LNCaP with IC50 in the low micromolar range (11 μM) without affecting VERO cells and PBMC proliferation (IC50 > 100 μM) indicating its low toxicity to normal cells.

 Please wait while we load your content...
Please wait while we load your content...