Synthesis of iminosugar derivatives presenting naphthyl and alkyl amine interacting groups and binding to somatostatin receptors†
Abstract
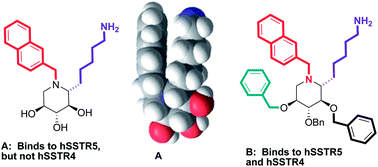
The synthesis of 1-deoxynojirimycin (DNJ) derivatives presenting a 2-naphthylmethyl and an alkyl amino side chain from L-sorbose is described. The synthetic derivatives were tested for their ability to inhibit the binding of somatostatin-14 to human recombinant somatostatin receptors (hSSTRs). One DNJ derivative showed selective binding for hSSTR5 over hSSTR4. The presence of benzyl groups and acetates on the oxygen atoms of the iminosugar scaffold led to increased affinity for both hSSTR5 and hSSTR4. Ligand-lipophilicity efficiencies (LLEs) are calculated for the iminosugar derivatives. The LLE values are significantly higher for iminosugar derivatives where hydroxyl groups are not protected, as compared to where they are benzylated. This indicates that leaving hydroxyl groups free or avoiding the use of multiple benzyl groups could be important for drug discovery research based on sugar scaffolds.

 Please wait while we load your content...
Please wait while we load your content...