Synthesis, anticancer evaluation and docking study of vadimezan derivatives with carboxyl substitution†
Abstract
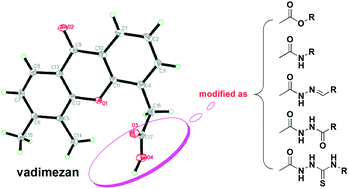
A series of xanthone analogues modified from vadimezan 6 with carboxyl substitution were synthesized as esters, amides, arylidene hydrazides, diacylhydrazides and acyl thiosemicarbazides, and their structures were confirmed by IR, 1H NMR, MS, HRMS or elemental analysis. The in vitro anticancer activities were evaluated by the MTT method. It was found that compounds 8f, 8g and 10e were effective against A549 with an IC50 at 10.8 μM, 9.4 μM and 11.5 μM respectively, and that 8e was effective against HL-60 with an IC50 at 4.6 μM. Compounds 8f–h showed a significant inhibitory effect on HUVEC growth and migration in vitro, among which 8h inhibited HUVEC growth with an IC50 at 6.4 μM and HUVEC migration by 67.6% and 89.7% at 2.5 μg mL−1 and 10 μg mL−1 respectively. More spectacularly, docking study indicated that compound 8h might target the ATP binding site of VEGFR2. In addition, compounds 8a, 8f–h exhibited moderate in vivo antitumor efficacy against the S180 xenograft in ICR mice by 22.4–29.6% tumor weight inhibition.

 Please wait while we load your content...
Please wait while we load your content...