Heterocycle-fused lupane triterpenoids inhibit Leishmania donovani amastigotes†
Abstract
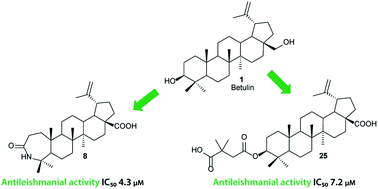
The synthesis of heterocyclic betulin derivatives and their activity against Leishmania donovani is reported. Betulonic acid was used as a versatile intermediate. Several different fused heterocycles were introduced at the 2,3-position of the lupane skeleton including isoxazole, pyrazine, pyridine, indole and pyrazole rings. Also the 28-position was modified. Three compounds, 5, 8 and 25, showed low micromolar activity with IC50 values of 13.2, 4.3 and 7.2 μM, respectively. Compound 8 showed the best activity and selectivity, and its activity was tested on infected macrophages using a concentration, 5 μM, where no macrophage toxicity was exhibited. Interestingly, the activity of compound 8 on axenic amastigotes and Leishmania-infected macrophages was similar.

 Please wait while we load your content...
Please wait while we load your content...