Synthesis and biological evaluation of new conformationally restricted S-DABO hybrids as non-nucleoside inhibitors of HIV-1 reverse transcriptase†
Abstract
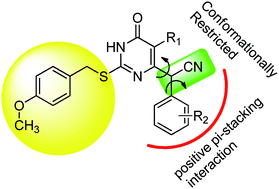
A series of conformationally restricted dihydro-alkylthio-benzyl-oxopyrimidine (S-DABO) hybrids, which combined the structural features of C6-α-methylbenzyl-thio-DABOs (α-methyl-S-DABOs) and C6-α-cyanobenzyl-thio-DABOs (CN-S-DABOs), has been synthesized and biologically evaluated for their anti-HIV activity against wild-type HIV-1 strain IIIB, double RT mutant (K103N + Y181C) strain RES056 and HIV-2 strain ROD in MT-4 cell cultures. Most of these compounds exhibited inhibitory activity (wild-type) within the range of EC50 values from micromolar to nanomolar. Among them, compound 1s displayed the highest anti-HIV-1 activity with an EC50 value of 91 nM and a selectivity index (SI) of 548, which was more potent than zalcitabine and comparable to nevirapine and delavirdine in the same assay. The HIV-1 reverse transcriptase inhibitory (RT) assay confirmed that these conformationally restricted S-DABO hybrids targeted HIV-1 RT. The preliminary structure–activity relationship (SAR) and molecular docking analysis of this new series of conformationally constrained CN-S-DABO hybrids were also investigated.

 Please wait while we load your content...
Please wait while we load your content...