Improved cyclopropene reporters for probing protein glycosylation†
Abstract
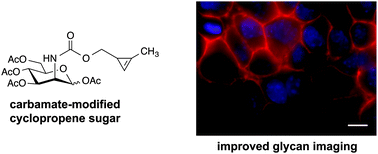
Cyclopropenes have emerged as a new class of bioorthogonal chemical reporters. These strained rings can be metabolically introduced into target biomolecules and covalently modified via mild cycloaddition chemistries. While versatile, existing cyclopropene scaffolds are inefficient reporters of protein glycosylation, owing to their branched structures and sluggish rates of reactivity. Here we describe a set of cyclopropenes for the robust detection of glycans on cell surfaces and isolated proteins. These scaffolds comprise carbamate linkages that are compatible with cellular biosynthetic pathways and exhibit rapid cycloaddition rates. Furthermore, these probes can be used in tandem with other classic bioorthogonal motifs—including azides and alkynes—to examine multiple biomolecules in tandem.
- This article is part of the themed collection: 2014 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...